SAM.gov Experiencing Unexpected Outage and Generating Mistaken Emails as of March 8, 2023
Client Alert | 11 min read | 03.08.23
As of the morning of March 8, 2023, SAM.gov is experiencing an unexpected outage and the system also appears to be generating false emails. Until this issue is resolved, companies and administrators should consider refraining from clicking on any links or email addresses within any SAM.gov email received until the system is confirmed as fully operational.
At this time, the Federal Service Desk website has confirmed both the outage and mistaken emails:
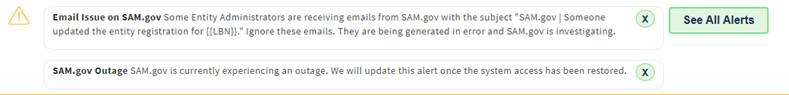
The emails described above appear to have at least in some instances identified various registration changes made by a foreign email address to one of the recipient’s SAM registration.
Updates on the status of the SAM email and outage issues will be provided through the Federal Service Desk.
Contacts
Insights
Client Alert | 4 min read | 02.20.26
SCOTUS Holds IEEPA Tariffs Unlawful
On February 20, 2026, the Supreme Court issued a pivotal ruling in Trump v. V.O.S. Selections, negating the President’s ability to impose tariffs under IEEPA. The case stemmed from President Trump’s invocation of IEEPA to levy tariffs on imports from Canada, Mexico, China, and other countries, citing national emergencies. Challengers argued—and the Court agreed—that IEEPA does not delegate tariff authority to the President. The power to tariff is vested in Congress by the Constitution and cannot be delegated to the President absent express authority from Congress.
Client Alert | 7 min read | 02.20.26
Section 5949 Proposed Rule Puts the FAR Council's Chips on the Table
Client Alert | 5 min read | 02.20.26
Trump Administration Pursues MFN Pricing for Prescription Drugs
Client Alert | 4 min read | 02.19.26
Proposed NY Legislation May Mean Potential Criminal Charges for Unlicensed Crypto Firms




