Is it Allowed? Companies Face New Challenges with an Ever-Evolving List of “Off Limits” Ingredients in Cosmetic Products
Client Alert | 14 min read | 05.07.24
As we have previously discussed, in recent years, consumers have increasingly demanded “cleaner” beauty products and more transparency in product labeling. In addition to the FDA’s increased authority to regulate cosmetics under MoCRA, a number of states have now taken steps to regulate ingredients in cosmetics by limiting, and in some instances even banning, the use of certain ingredients that may be potentially harmful or toxic.
This web of complex and evolving regulations can be challenging for companies that manufacture and sell cosmetic products to track and follow to ensure compliance, especially when their products may be sold nationwide. Below is a May 2024 survey that may be helpful to companies in the process of untangling the current regulatory framework.
By way of background, the vast majority of states (39 of 50) have passed legislation that broadly prohibits (1) manufacturing and selling cosmetics that contain ingredients that allegedly can cause injuries and (2) providing false or misleading information on product labeling or packaging.[1]
Recent state legislation, however, goes one step beyond these regulations. As of May 1, 2024, the following states have enacted or proposed legislation limiting the manufacture, sale, and distribution of cosmetic products containing certain ingredients, including, but not limited to, chemicals like per- and polyfluoroalkyl substances (PFAS):
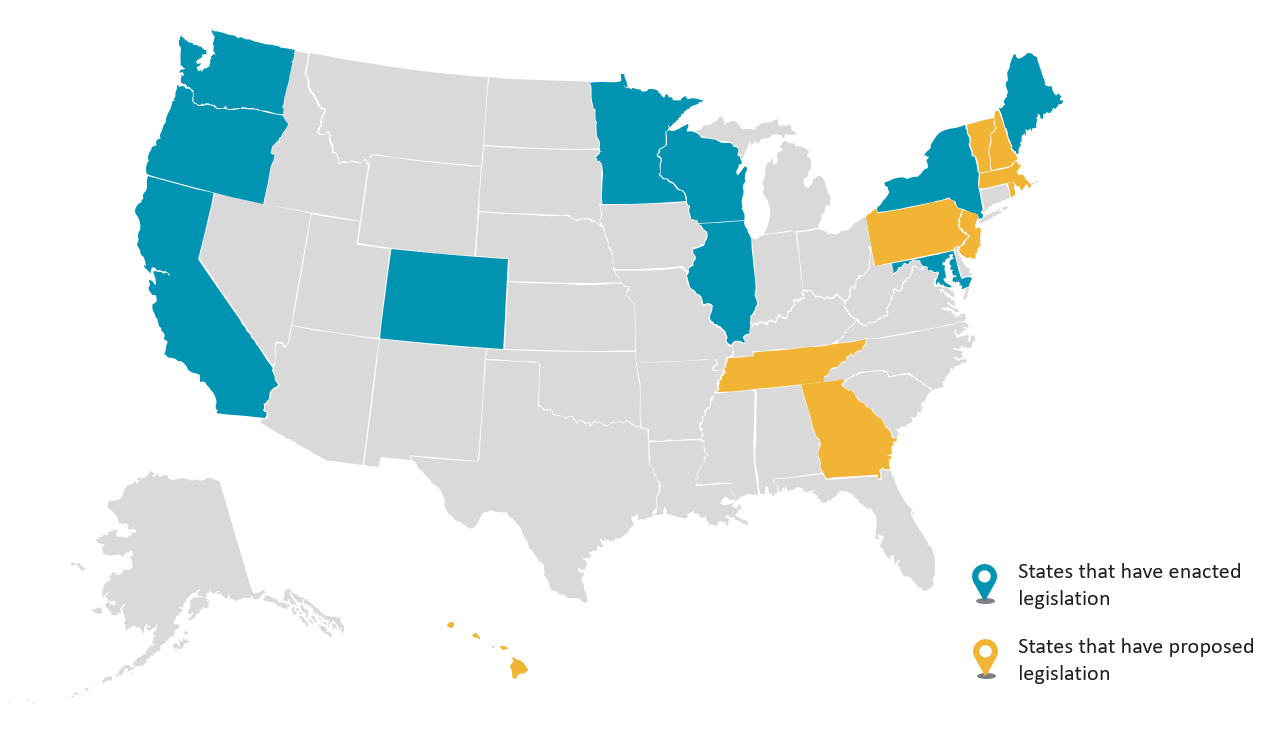
This recent legislation breaks down as follows:
|
ENACTED legislation banning or limiting use of PFAS in cosmetics |
PROPOSED legislation banning or limiting use of PFAS in cosmetics |
ENACTED legislation banning or limiting use of certain ingredients other than PFAS in cosmetics |
PROPOSED legislation banning or limiting use of certain ingredients other than PFAS in cosmetics |
|
|
|
|
Still unclear as to what is in and what is out? Our team has prepared a List of Banned Ingredients by State—a summary of the ingredients banned or restricted in each state, which we will monitor and update on a routine basis.
[1] States that have such legislation on the books include: Alabama, Alaska, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Missouri, Montana, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Vermont, Virginia, Washington, West Virginia, and Wyoming.
Contacts
Insights
Client Alert | 4 min read | 02.05.26
EU–Brazil Mutual Adequacy: A Milestone for Global Data Flows and Latin America’s Digital Positioning
On January 27, the EU and Brazil announced their positive determination on the mutual adequacy of Brazil’s and the EU’s data privacy frameworks — confirming the growing importance of transatlantic data transfers and the EU-Mercosur relationship. This adequacy decision, while not formally tied to the EU-Mercosur trade negotiations, is a historic development that can facilitate cross-border data transfers and fuel shared economic growth driven by data-centered service sectors.
Client Alert | 4 min read | 02.04.26
DOJ Antitrust Division Issues First-Ever Award Under Whistleblower Rewards Program
Client Alert | 4 min read | 02.04.26
New York District Court Confirms Insurance Coverage Must Mean Something
Client Alert | 13 min read | 02.04.26






